Species Adaptation in the Hemoglobin Molecule
A Seminar by Max Perutz
- 1985-Mar-20
These captions and transcript were generated by a computer and may contain errors. If there are significant errors that should be corrected, please let us know by emailing digital@sciencehistory.org.
Transcript
00:00:50 It's my pleasure, a big pleasure to welcome to the University of Chicago today Dr. Max Perutz of the MRC Laboratory of Electrobiology in Cambridge.
00:01:13 Some people are said to be of such distinction that they need no introduction, if ever that were the case, it's true here.
00:01:21 However, it's a really good opportunity to introduce anyone of Max's distinction, I'm going to introduce him anyway.
00:01:30 Max started his scientific career as an undergraduate in chemistry at the University of Vienna, and in 1936 went to Cambridge to take up graduate school.
00:01:41 Now he originally wanted to work on amino acids with Colin Hopkins, but his mentor in Vienna, Herman Mark, pre-polymer chemist,
00:01:50 had just returned from Cambridge and was all aglow with reports of X-ray diffraction patterns from crystals of protein,
00:01:59 having been taken by J.D. Bernal and Dorothy Crowfoot, subsequently Dorothy Crowfoot Hodgkin, and their collaborators in Cambridge.
00:02:09 They were so excited about the prospect of being able to visualize protein molecules in detail so that they could figure out how they worked,
00:02:19 and they transmitted this enthusiasm to Mark, who signed Max up to work with Bernal.
00:02:26 Not that Max knew about this until he paid a call on him.
00:02:31 So Max went to Cambridge and began his work there on protein crystallography, and he began working on the hemoglobin molecule.
00:02:42 Well there was a lot of intervening history and a lot of hard work, nineteen- in the war years as well.
00:02:48 We'll jump ahead to 1947, when Max became director of the MRC unit at the Cavendish lab.
00:02:56 He had a giant staff of one person, John Kendrew.
00:03:00 Well the lab grew, and you all know the luminaries who came there.
00:03:05 And in 1962 they moved to their new residence on Hills Road with a much expanded staff.
00:03:14 In 1962 their work was acknowledged by the laboratories winning four Nobel Prizes in one year.
00:03:21 Max and John Kendrew in chemistry for their work on the hemoproteins, the crystallography of the hemoproteins,
00:03:27 and some young fellows by the name of Watson and Crick in collaboration with Wilkins for the structure of DNA.
00:03:35 Well since that time that laboratory has manifested itself in a manner that I believe no others have in the area of biology, certainly at the molecular level.
00:03:48 Having won in total seven Nobel Prizes to a building about half the size of this one, isn't that quite an accomplishment?
00:03:56 And it's been under the directorship of Max.
00:03:58 Meanwhile, about five years ago Max turned over the directorship to Sydney and it was a kind of revert.
00:04:05 He re-entered into his already active program on hemoglobin structure-function relationship.
00:04:10 He's going to tell us today about one of the areas that he's been studying and that's the species adaptation in the hemoglobin molecule.
00:04:40 Can you hear me at the back?
00:05:09 Yes.
00:05:10 Yes, good.
00:05:13 Levantin complained in an article in the Scientific American a few years ago that it has proved remarkably difficult
00:05:23 to get compelling evidence for changes in enzymes brought about by selection, not to speak of adaptive changes.
00:05:32 Fortunately, such evidence has recently been gathered for hemoglobin, whose response to different chemical stimuli varies widely in vertebrates living in different environments.
00:05:46 So one asks oneself, how did this adaptation come about?
00:05:50 Is it the result of changes in tertiary or paternal structure of the hemoglobin molecule?
00:05:57 Or has it been brought about by the gradual accumulation of minor mutations, each producing a small change in chemical affinity?
00:06:05 Or there are fewer amino acid substitutions in T-positions, each producing a large change in chemical affinity.
00:06:13 Have similar changes in affinity in different animal species been brought about by the same amino acid substitutions or the different ones?
00:06:25 And finally, are most amino acid substitutions between species functionally significant?
00:06:35 And have they evolved by Darwinian selection?
00:06:41 Or are they caused by random fixation of neutral or almost neutral mutations?
00:06:46 Are they results merely of genetic drift?
00:06:51 Now, by studying the adaptation of the hemoglobin molecule, one can at least begin to answer some of these fundamental questions.
00:07:01 Now, perhaps I should introduce hemoglobin, because we won't all be familiar with it.
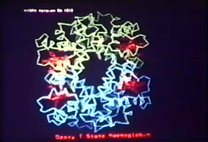
00:07:09 And for this purpose, I would like to have the first stereoslide.
00:07:18 Have you all got stereoglasses, or stereoglasses have your neighbors got them?
00:07:31 What about these? Are these pairs?
00:07:35 Yes, I think you do have a couple of them.
00:07:40 So, this shows a...
00:07:50 Not very clearly visible from the side.
00:07:54 This shows the stereo view of my own hemoglobin model, which in its four subunits,
00:08:04 the two white subchains representing the alpha chains and the black ones, the beta chains,
00:08:12 and the hemes being in the pockets on the surface of the molecule.
00:08:19 And here is the position of the reactive start-hybrid group of hemoglobin,
00:08:25 at residue 93 beta, which we shall be talking about.
00:08:30 And going through the center of the molecule from its top, is a two-fold symmetry axis,
00:08:36 so that a rotation of 180 degrees brings this black chain of the congruence to its opposite.
00:08:47 The most important feature of this molecule is its change of structure on taking up oxygen.
00:08:59 This is the oxy-hemoglobin structure.
00:09:02 On loss of oxygen, one pair of subunits, one white and one black chain,
00:09:09 rotates relative to the other pair by 15 degrees,
00:09:13 thus widening the slit between the two beta chains at the bottom
00:09:18 and opening a binding site for the allosteric regulator 2,3-biphosphoglycerate.
00:09:24 So, hemoglobin alternates between two structures,
00:09:28 one with an oxy- or T-structure with a low oxygen affinity
00:09:32 and a high affinity for protons, chlorides, carbon dioxide,
00:09:37 and in mammals 2,3-biphosphoglycerate,
00:09:41 the other structure in which these relative affinities are reversed.
00:09:48 The oxygen affinities of the two alternative structures in mammals
00:09:55 differ by the equivalent of 3 kilocalories per mole,
00:10:01 and that is usually referred to as the free energy of heme-heme interaction.
00:10:06 So, so much for this introduction,
00:10:09 and now we can stop the stereos and look at the first monoslide, please,
00:10:17 which shows the tertiary structure of the hemoglobin molecule
00:10:25 and the notation that is used to designate the different residues.
00:10:32 This was introduced by Kangu from myoglobin and has been very useful
00:10:37 because the number of amino acid residues in the hemoglobin of different animal classes differs,
00:10:45 say, the alpha chain in human hemoglobin has 142 residues and in fish 140 - sorry, in humans 141 and in fish 142,
00:10:57 but insertions and deletions occur at the corners of non-helical segments
00:11:05 and the number in the helical segment tends to be constant.
00:11:09 So we call the helical segments A, B, C, D, etc. to H,
00:11:15 and the non-helical one, the first one we call NA for the segment with the amino end,
00:11:25 and then C, D, and so on until we come to GH here,
00:11:31 and finally to HC, which designates several non-helical residues at the carboxyl end.
00:11:41 The heme you see in this pocket here is sandwiched between two histidines,
00:11:47 one covalently linked to the R, which is residue F8,
00:11:52 to its residue along helix F,
00:11:55 and the other, the distal one, which is hydrogen bonded to the linked oxygen when it's there,
00:12:04 which is E7, so it's the seventh residue along the E helix.
00:12:10 The next slide shows you a view of the hemoglobin molecule,
00:12:16 the same one that we saw before,
00:12:19 from the, along the direction of the super-extremity axis,
00:12:24 which runs through the central cavity as you see here,
00:12:28 and there you see the amino termini of the alpha chain,
00:12:33 which have some important part to play in function.
00:12:40 Right, now, the next slide shows you a logarithmic plot
00:12:47 of two pythagorean-oxygen equilibrium curves of human hemoglobin
00:12:51 across on the x axis, the partial pressure,
00:12:54 the log of the partial pressure of oxygen on the ordinate,
00:12:58 the log of Y over 1 minus Y,
00:13:02 where Y is the fractional saturation with oxygen.
00:13:07 So these equilibrium curves are sigmoid,
00:13:11 because the binding of oxygen is cooperative,
00:13:15 and they are pH-dependent,
00:13:17 so that the oxygen affinity rises with increasing pH,
00:13:26 with lowering hydrogen ion concentration,
00:13:31 so oxygen and hydrogen ions are antagonistic,
00:13:35 and there's a profound physiological purpose for this,
00:13:41 because hemoglobin is a two-way respiratory carrier,
00:13:45 it carries oxygen from the lungs to the tissues,
00:13:48 and it promotes the return transport of carbon dioxide
00:13:52 from the tissues back to the lungs,
00:13:54 and it does that by picking up hydrogen ions on loss of oxygen.
00:14:02 From loss of oxygen, hemoglobin mops up the hydrogen ions
00:14:07 that are liberated in the reaction of carbon dioxide and water
00:14:12 to form bicarbonate,
00:14:14 thus shifting the mass equilibrium towards bicarbonate ions,
00:14:20 which are soluble and can be transported back to the lungs in the serum.
00:14:26 In the lungs, hemoglobin takes up oxygen,
00:14:32 liberates hydrogen ions,
00:14:35 which pushes the equation back to the left
00:14:39 towards carbon dioxide and water,
00:14:42 and carbon dioxide is exhaled.
00:14:44 But moreover, you see that the liberation of lactic and carbonic acids in the tissues
00:14:52 lowers the oxygen affinity
00:14:54 and so promotes the liberation of oxygen.
00:14:58 Now, this effect of the H-dependent of the oxygen equilibrium
00:15:06 and the antagonistic effects of oxygen and hydrogen ions
00:15:12 are known as the Bohr effect,
00:15:14 after Christian Bohr,
00:15:16 and many physiologists who discovered them.
00:15:21 Now, so much then for the human.
00:15:36 Some years ago, an Italian chemist, Maurizio Brunori,
00:15:45 showed me what the equivalent curves are in the trout.
00:15:50 Can I have the next slide, please?
00:15:54 So, here again, you see we have the log of the partial pressure of oxygen,
00:15:59 and instead of y, it holds the fractional separation x bar,
00:16:04 but it's the same thing.
00:16:06 And here are the equilibrium curves.
00:16:12 Note that only at this middle pH, which is 7.15,
00:16:19 does the equilibrium curve resemble the human one.
00:16:26 At alkaline pH, it's no longer sigmoid,
00:16:31 and at acid pH, it becomes side-basic,
00:16:35 and the shift is far to the right,
00:16:38 so that the influence of pH on the equilibrium curve
00:16:49 is enormously exaggerated in this trout hemoglobin.
00:16:56 But that's not all.
00:16:58 Trout actually has two kinds of hemoglobin,
00:17:01 one which is enormously dependent,
00:17:04 and another with a sigmoid oxygen equilibrium curve
00:17:08 that is totally independent of pH and all the other co-factors
00:17:12 which shift the curve in human hemoglobin.
00:17:18 Well, Brunori, with an old friend, came along and said,
00:17:23 how do you explain this?
00:17:25 And I said, I can't do it if you don't show me a sequence.
00:17:31 Now, before I show you the sequence,
00:17:36 let me explain what its use is to the fish.
00:17:44 The effect to which Brunori drew my attention
00:17:48 was originally discovered by an American geologist,
00:17:52 Ruth, working at the Duke University
00:17:56 marine biology laboratory in Beaufort, North Carolina,
00:18:01 in the 1930s,
00:18:04 and who showed that fish use this
00:18:09 to fill their swim bladder with oxygen.
00:18:13 My next slide shows you a diagrammatic sketch
00:18:18 of the anatomy of the swim bladder
00:18:22 which has attached to it what is called here a secretory epithelium,
00:18:29 in effect, a gland which secretes lactic acid into the blood
00:18:34 and so acidifies it.
00:18:37 And because the oxygen affinity is so strongly pH-dependent,
00:18:45 the secretion of lactic acid leads to the liberation of oxygen in the swim bladder.
00:18:51 But again, there's more to it, because attached to the swim bladder
00:18:56 is a beautiful counter-current system of small capillaries
00:19:00 where a gradient of increasing lactate concentration
00:19:09 is converted to a gradient of decreasing oxygen saturation of the hemoglobin.
00:19:16 In other words, this is a device for transferring the unused oxygen of the veins to the arteries
00:19:30 so that more of the oxygen that is carried can be secreted into the swim bladder.
00:19:38 The next slide shows you a microscope section through this organ
00:19:46 and you see that, well, you can't see it because it is closed,
00:19:52 that each of these arteries tends to be surrounded by veins
00:19:57 and vice versa so that you get a perfect exchange.
00:20:03 Now, since then people have discovered that there is another such system
00:20:08 at the back of the retina of fish ensuring secretion of oxygen into the eye.
00:20:17 So the fish use this rudiment then both for regulating their buoyancy
00:20:28 and improving their vision.
00:20:33 So, as I told you, Brunori asked me how do you explain this
00:20:40 and at the fullness of time the amino acid sequences of several fish hemoglobins
00:20:45 became known and were [carved as the goldfish] of these two components of trout hemoglobin
00:20:54 and of an American fish called [fox].
00:20:59 So, I looked at these and they seem very unpromising
00:21:10 because about half the amino acid residues of human hemoglobin differ from the fish.
00:21:20 The hemoglobin amino acid sequences are surprisingly variable.
00:21:27 You know, if you look at all the hemoglobins of vertebrates and de-vertebrates
00:21:33 there are in fact only two invariant residues.
00:21:36 One is the proximal histidine which is essential for the oxygen binding
00:21:42 and the other is the phenylalanine which is in the fish's [pockets].
00:21:46 And all the other residues are exchangeable.
00:21:50 So, in the differences between trout and human, there is, as I say, about 140.
00:21:59 So where do you start?
00:22:01 Well, as often in science, you have to take your courage into your hand
00:22:08 and decide you're going to look at these amino acid mutations one by one
00:22:13 and ask yourself for each one of them whether that could possibly have produced
00:22:19 this root effect, this enormous increase in the pH dependence of the oxygenic hemoglobin.
00:22:26 And when you do that, you find that the vast majority of the mutations are in fact conservative ones
00:22:32 of non-polar residues in the interior or polar residues in the exterior
00:22:39 which clearly have no particular function.
00:22:42 But then one particular replacement caught my eye, which I now want to tell you about.
00:22:48 And this is of the reactive cysteine in mammals by serine in all these fish.
00:22:58 The next slide shows you just the position of the reactive H-group again,
00:23:07 which is here in the model.
00:23:09 And in the next slide, the function of the human hemoglobin.
00:23:18 So, here you have a diagrammatic sketch showing the heme and the human deoxyhemoglobin
00:23:24 and the human oxy.
00:23:26 The deoxy, the R is out of the frame of the forefront.
00:23:30 Here's the proximal histidine residue, F8.
00:23:34 And the next one, F9, is this reactive cysteine,
00:23:39 which in deoxyhemoglobin is surrounded by the C-terminal histidine
00:23:45 that forms a salt bridge with aspartate
00:23:52 and with the lysine C-prime alpha.
00:24:00 Now, in human oxyhemoglobin, the salt bridge is broken
00:24:06 and the C-terminal histidine instead forms a salt bridge with the lysine here.
00:24:15 The idazole accepts the hydrogen bond from its own end, which is not yet shown here.
00:24:24 So, this changing conformation of the C-terminal histidine
00:24:31 turns out to be responsible for about half the Bohr effect in human hemoglobin
00:24:37 because salt bridge with aspartate reduces decay to eight
00:24:43 and the rupture of the salt bridge lowers its decay to about seven.
00:24:53 Right. Now, what I noticed then was that in restriction hemoglobin,
00:24:58 this SH group is replaced by serine.
00:25:03 In human hemoglobin, it seems to act merely as a spacer.
00:25:13 That is to say, the cysteine SH forms on the very weak hydrogen bond.
00:25:22 For instance, if you look at the neutron refraction structure of cysteine itself,
00:25:28 then the SH group is in contact with the carboxylate of a neighboring cysteine molecule,
00:25:37 but the distance from the hydrogen to the oxygen is no larger than the sum of the Van der Waals radii.
00:25:44 Whereas if you did the same with a serine,
00:25:47 you'd probably find very strong hydrogen bonding,
00:25:50 the distance being several tens of angstroms shorter than the sum of the Van der Waals radii.
00:25:56 Now, the next slide shows you the position in carp,
00:26:02 where, as I said, we have a serine in this position.
00:26:07 So, what I did was take the model of human hemoglobin
00:26:12 and simply replace the cysteine by a serine.
00:26:17 And when I did that, I found that the serine can donate the hydrogen bond to this terminal carboxyl,
00:26:26 one that would be oxygen that would not bond with the live thing,
00:26:30 and that it can accept the hydrogen bond from the NH of this T-terminal histidine
00:26:38 in the deoxy or T-structure.
00:26:41 In the oxy or R-structure, these bonds would be broken.
00:26:45 So, I argued then that if [when the] salt bridge is closed,
00:26:50 these bonds would contribute something between 1 and 2 kilocalories per mole of stabilization energy to the T-structure,
00:27:04 and this may be responsible for the enormously reduced oxygen affinity and acid pH,
00:27:15 because acid pH stabilizes that sulfate.
00:27:19 Well, that was just an idea, but how do you find out whether there is anything in it?
00:27:28 Well, one way in which Kilmartin had tested,
00:27:42 and my colleague Kilmartin had tested the original Bohr mechanism,
00:27:48 was to leave away the T-terminal histidine,
00:27:54 and see how it affected the Bohr effect, and the T-terminal capacity.
00:28:00 Now, in carp, this was much more difficult,
00:28:03 in fish hemoglobin because you can't stick anything into alpha and beta chains,
00:28:07 but Lawrence Parker at the University of Lincoln in Nebraska succeeded in doing this,
00:28:13 he cleaved away the T-terminal histidine,
00:28:16 and measured the influence of that cleavage on the oxygen equilibria.
00:28:26 Now, the next slide, please.
00:28:29 So, here the full curve of naked carp hemoglobin,
00:28:38 and there you see the curve at pH 7, and at pH 6, and pH 9,
00:28:50 and the working curves show that this is carp hemoglobin,
00:28:56 in which the root effect, Bohr effect is greatly reduced.
00:29:04 So, this is a kind of negative test, but I hope that we can soon have a positive one.
00:29:14 It comes from my colleague Kiyoshi Nagai,
00:29:22 has developed a method of cloning human hemoglobin in E. coli,
00:29:28 so that we can now subject it to a redirected mutagenesis,
00:29:33 and convert the cysteine in position 8-9 to a serine,
00:29:41 and see what influence it will have on the oxygen equilibria.
00:29:46 So, in the fullness of time, I hope that we will see a communication,
00:29:53 in Nature, on turning man into fish,
00:29:57 and see what the result of this experiment is going to be.
00:30:04 So, this then is one of the adaptive mechanisms.
00:30:12 Now, another one, for reasons which are not so clear, is this.
00:30:22 In human hemoglobin, the oxygen affinity is regulated by 2,3-diphosphoglycerate.
00:30:29 So, the next slide shows you the binding site in human hemoglobin.
00:30:36 There is the diphosphoglycerate, which is phosphate, which is carboxylate,
00:30:41 and in deoxyhemoglobin, there is a constellation of 8 complementary cationic groups,
00:30:49 which bind to the diphosphoglycerate anion.
00:30:53 So, you see these two histamines, these two lysines, this alpha-amino group.
00:31:00 In the transition to the oxygen structure, these two segments, the ES segments,
00:31:09 move apart, move together, and the alpha-amino groups move apart,
00:31:15 so that the complementarity is lost, and the DPP dissociates.
00:31:19 The next slide shows you the situation in oxyhemoglobin.
00:31:25 You see these two valines are now much further apart,
00:31:30 and the lysines are closer together.
00:31:33 And here you see another way to do lysine, a hundred and forty-four.
00:31:39 So, human hemoglobin responds strongly to diphosphoglycerate,
00:31:47 but very weakly to ATP or GTP.
00:31:52 In fish, it is reversed.
00:31:54 They show a very strong response to GTP, about half that response to ATP,
00:32:01 and a very weak one to diphosphoglycerate.
00:32:05 So, in the red cells, there is a very high concentration of the two triphosphates,
00:32:13 purine triphosphates, ATP and GTP.
00:32:17 And so, again, the physiologist asks me,
00:32:22 how can you convert these affinities?
00:32:26 What can reverse the relative affinities,
00:32:31 or change it from diphosphoglycerate to purine triphosphate?
00:32:37 So, again, the sequence gives a clue.
00:32:43 The sequence shows that in these fish hemoglobin,
00:32:47 this histidine 2 here is referred to.
00:32:51 It's replaced either by an aspartate or a glutamate.
00:32:55 We have an acid here.
00:32:57 And this histidine 143 is replaced by a stronger base,
00:33:03 by arginine.
00:33:06 Moreover, Allen Edmondson showed many years ago
00:33:10 that treatment of the alpha-amino group entirely abolishes the affinity for ATP.
00:33:22 So, the alpha-amino groups are clearly involved.
00:33:26 Right.
00:33:27 Now, again, I said, and this is a crucial point,
00:33:31 I said, let us assume that the tertiary-quaternary structure
00:33:36 of 4-carbon hemoglobin is exactly the same as that of human hemoglobin.
00:33:42 And merely replace one side chain by another.
00:33:50 We merely remove this histidine by a glutamate,
00:33:54 that histidine by arginine,
00:33:57 and then introduce a molecular model of ATP and see whether it fits.
00:34:04 Now, may I have the next stereo slide, please?
00:34:26 So, what have we got here?
00:34:29 Here's the alpha-amino group of the valine 1-alpha.
00:34:35 Here's this glutamate 2.
00:34:38 And there's lysine 82, which is conserved.
00:34:42 And here's the arginine, which was 143 in humans.
00:34:50 So, what I did is I simply took an extended model of ATP
00:34:56 and put it purely in the anti-conformation.
00:35:00 I said, we must have the gamma phosphate linked to the valine 1-alpha-amino group
00:35:11 as our experiment would have worked if it did.
00:35:16 And clearly, the glutamate [also had to be bound]
00:35:23 with the amino group of the adenine.
00:35:26 And so that's all I did, and it fitted in perfectly.
00:35:33 Now, next, I wondered how could I fit in TTP,
00:35:44 and how could I explain that the affinity of TTP,
00:35:49 the binding constant, is twice that of ATP.
00:35:58 And first, I was perplexed, because in TTP,
00:36:04 this amino group here is replaced by a carbonyl.
00:36:08 But then, with the help of an NMR experiment,
00:36:12 which I shall show you presently, I dropped the [fluid]
00:36:18 and simply turned this model.
00:36:22 Well, I fixed a guanine here and turned the entire model
00:36:27 by 180 degrees about this long axis.
00:36:32 And the next slide shows you the result.
00:36:37 May I have the next stereo
00:36:39 please?
00:36:45 So this needs adjusting, so wait a little bit before you look at it.
00:36:53 I think, isn't it a long?
00:36:56 It's vertical adjustment.
00:36:57 It's vertical adjustment.
00:36:58 It's a long way out of space.
00:37:01 Adjust it.
00:37:05 That's better.
00:37:06 Now it's right.
00:37:07 Good.
00:37:08 So, here you see the TTP again, and you see that
00:37:22 its amino group forms a hydrogen bond again with glutamate.
00:37:29 And this time, one of the hydroxides of the ribose
00:37:35 is in a position to form a hydrogen bond
00:37:39 with the alpha-amino group of the valine.
00:37:42 So the TTP is bound by one additional hydrogen bond,
00:37:47 and this accounts, lastly, for its greater affinity
00:37:52 compared to TTP.
00:37:55 Right, thank you.
00:38:04 So, can we have the next mono slide?
00:38:09 In fact, the job wasn't as easy as that.
00:38:13 And my first proposal for the TTP binding
00:38:16 was shown to be incorrect by NMR, [as started].
00:38:24 I collaborated with two young NMR specialists
00:38:28 at the National Institute of Medicine in London,
00:38:31 Marius Clore and Angela Gronenborn,
00:38:34 and they determined the combination of the bound ATP and TTP
00:38:43 by the time-dependent transferred nuclear overhauling effect,
00:38:49 which depends on a fast exchange between free and bound cofactor.
00:39:00 You get, what you do is you irradiate the resonance of one
00:39:07 of the hydrogens and determine the interaction
00:39:14 with another hydrogen as a function of time.
00:39:21 So down here, you see the notation for ATP,
00:39:28 and what we wanted to know was this.
00:39:31 We wanted to know whether the corrin ring is
00:39:34 in anti- or syn-position relative to the rival.
00:39:39 The way you see it here, it is in anti-position,
00:39:42 but if you turn it by 180 degrees,
00:39:45 about this ends in bond, which would be syn.
00:39:48 Sorry, is it in syn?
00:39:52 Yes, I think you were right, it's in syn.
00:39:57 So, what we did then was to irradiate the H1 prime,
00:40:06 that would be this proton,
00:40:09 and then determine its effect on the resonance of H8,
00:40:16 which you see here, and then irradiate the H2 prime,
00:40:23 determining the resonance of each dehydrogen here,
00:40:29 dehydrogen there, and the H1 prime, and so on.
00:40:36 So the object was to see whether it was the hydrogen at C2
00:40:44 or the hydrogen at C8, which was next to the hydrogen
00:40:49 of the ribose ring.
00:40:52 Now, when the hydrogens are close together,
00:41:00 the curve is convex like that.
00:41:05 When they are far apart, it is concave like this.
00:41:09 So what this experiment tells us is that H2
00:41:14 is within about 3 or 3.5 angstroms of H2 bar.
00:41:21 So like so.
00:41:23 And that it is distant from H8.
00:41:28 H3 bar is close to H2.
00:41:35 And H1, again, is close to H2, but distant from H8.
00:41:42 The result told us that the purine ring
00:41:45 is an anti-conformation relative to the rivals
00:41:50 in both ATP and GTP.
00:41:52 And this helped me to get the model right.
00:41:56 The next slide shows you another NMR experiment with P31,
00:42:01 where Clore and Gronenborn tried to find out
00:42:05 whether the phosphate chain is extended or coiled.
00:42:09 And the result showed that the resonances were similar
00:42:14 to that of 3-ATP, in which the chain is known to be extended
00:42:19 so that I got the conformation for that.
00:42:26 Now, I went into this, you see, to stress the point
00:42:30 that it is possible for a protein to change its affinity
00:42:38 from one protactor to a totally different protactor
00:42:43 without any alteration in chemical structure,
00:42:46 but merely by the replacement of a very few amino acids
00:42:50 in key positions, such as histidine 2,
00:42:54 which bound the internal phosphate of GTP
00:42:58 by glutamic acid, which instead formed a hydrogen bond
00:43:03 with the amino groups of adenine O1.
00:43:07 All right.
00:43:09 Now, there was one [hit] on all this.
00:43:14 It fell out rather nicely.
00:43:16 And that came from the other [chunk in the urine],
00:43:21 which had shown no dependence of its oxygen affinity
00:43:25 on either hydrogen ions or organic phosphate or CO2.
00:43:29 It was totally constant.
00:43:32 And in due course, Bossa, a colleague of Brunori's,
00:43:37 and [of Ron's], his colleagues, collaborators,
00:43:41 worked out the amino acid sequences
00:43:43 of these two hemoglobins, which are called
00:43:46 TROUT1 and TROUT4.
00:43:48 Now, one point that you would wonder about,
00:43:51 why should the fish want another hemoglobin
00:43:55 that shows no pH dependence in its oxygen equilibrium?
00:43:59 Well, I mean, you can't ask them,
00:44:02 but what the comparative physiologists believe is this,
00:44:09 that the trout is a very fast-moving fish,
00:44:13 and that during fast movement,
00:44:15 the pH in its skin will drop so low
00:44:19 that it can no longer take up enough oxygen
00:44:24 with this hemoglobin 4,
00:44:27 whose oxygen affinity drops so low with acid.
00:44:32 So the fish needs another hemoglobin in reserve,
00:44:36 which allows him to pick up oxygen under these conditions.
00:44:42 Now, then, you see, the question is,
00:44:47 if my ideas are right,
00:44:52 then all these key residues should be replaced
00:44:56 by, as it were, neutral ones in the TROUT1.
00:45:00 And I think the next slide shows
00:45:05 the amino acid sequences of human hemoglobin
00:45:11 in these key positions compared to trout.
00:45:16 So, first of all, let's look and see
00:45:20 what the C-terminal histidine does,
00:45:23 which contributes powerfully to the Bohr effect.
00:45:31 So it's histidine in human, and histidine in TROUT1,
00:45:34 but it's phenylalanine in TROUT4.
00:45:38 So there, you have only a van der Waals interaction
00:45:44 between the phenyl side chain and the helix H
00:45:52 to stabilize the key structure, but no hydrogen bond.
00:45:57 Now, then, there is this cysteine, F9,
00:46:07 which is here in TROUT4,
00:46:10 which is changed to alanine in TROUT1.
00:46:14 Then there's aspartate,
00:46:17 that forms the hydrogen bond with the C-terminal histidine,
00:46:20 that's the aspartate ST1,
00:46:22 that's glutamate in TROUT4,
00:46:25 but asparagine, an un-TROUT residue, in TROUT1.
00:46:31 So, so much, then, for the residues responsible for the root effect.
00:46:35 Now, let's look at those responsible for the binding of ATP.
00:46:40 A central role is played by this lysine, PH6.
00:46:45 You know, that's the one that's conserved in human and fish.
00:46:49 And we see in TROUT1 that's replaced by leucine.
00:46:53 The arginine, which was H21,
00:46:59 that's the one that was histidine in human,
00:47:02 arginine in TROUT4,
00:47:04 has been replaced by serine.
00:47:07 So, these two replacements are sufficient to account
00:47:13 for the loss of ATP activity in the TROUT1.
00:47:19 And that's all encouraging.
00:47:22 Now, there are other fish which have no swim bladder.
00:47:28 You see, the shark has no swim bladder,
00:47:31 it has to swim always to keep afloat.
00:47:38 If the shark stops swimming,
00:47:41 it drops to the bottom and can never swim.
00:47:46 And so, now, the shark hemoglobin
00:47:50 has, as you would expect from that,
00:47:53 only a weak Bohr effect.
00:47:55 There's no root effect right now.
00:47:57 So you ask yourself, how does this come about?
00:48:00 Well, first of all, the absence of the root effect
00:48:03 is explained by residue F9 being alanine.
00:48:08 You see?
00:48:10 So, no arginine bonds there.
00:48:14 And then, there is the C-terminal residue in histidine,
00:48:23 but there's now another residue, a lysine,
00:48:25 one term down, one thermophilic star from HB1,
00:48:30 to compete with the histidine for the hydrogen bond
00:48:35 which gets intubated,
00:48:37 so that it only weakens the Bohr effect.
00:48:42 And then, there's the lionfish, you know?
00:48:45 So the lionfish snaps up air, you see,
00:48:50 and absorbs it through its lungs,
00:48:54 so it uses its lungs for buoyancy,
00:48:58 so it doesn't have to need a swim bladder.
00:49:00 So again, we have no root effect.
00:49:05 Now, but if this...
00:49:09 Let's see how does this work.
00:49:12 If you have some therine here,
00:49:15 if you again have a second histidine there,
00:49:19 so this is H6,
00:49:21 to compete with the histidine there
00:49:24 for the solvent which can neutralize it.
00:49:32 So much then for the fish.
00:49:37 Now, what about amphibia?
00:49:42 You see, they don't really need a strong Bohr effect
00:49:48 because the pressure of carbon dioxide,
00:49:55 the blood of amphibia is so much lower
00:49:59 than in mammals, in terrestrial animals.
00:50:03 In humans, the PCO2 in the blood is 40,
00:50:08 in the human's blood is 40 millimeters, 44.
00:50:12 40 millimeters is quite big.
00:50:14 But in this amphibia,
00:50:17 it's only between 2 and 4 total
00:50:21 because of carbon dioxide,
00:50:24 that diffuses through their skin.
00:50:29 And so they, even if they live in water,
00:50:36 they don't, sorry,
00:50:38 so usually they don't have a very strong Bohr effect.
00:50:43 Xenopus, there is an exception.
00:50:46 And in the tadpoles,
00:50:49 the Bohr effect is actually reversed
00:50:52 so that the oxygen actually decreases with,
00:50:59 sorry, increases with increasing pH,
00:51:03 we see again, which we come with
00:51:05 in the case of phenylalanine.
00:51:09 Right, now, some years ago,
00:51:14 another one of these comparative physiologists
00:51:18 came along, this time a German, Christian Bauer,
00:51:21 who was in the habit of walking into an aquarium
00:51:26 and taking crocodiles by the tail,
00:51:29 pulling them into a plastic bag,
00:51:31 anaesthetizing them,
00:51:33 taking a blood sample from them
00:51:36 and measuring their oxygen equilibrium levels.
00:51:39 And by doing that,
00:51:40 he discovered that crocodilian hemoglobins
00:51:44 do not respond to any of the flow factors
00:51:47 that other vertebrates respond to,
00:51:49 but only to bicarbonate ions,
00:51:52 which has a dramatic effect on a crocodile's equilibrium.
00:51:56 So if I can have the next slide, please.
00:52:00 This shows you a comparison of the response
00:52:07 to carbon dioxide of horse hemoglobin,
00:52:10 the two broken lines,
00:52:12 and of canine hemoglobin.
00:52:14 So, in the crocodilians,
00:52:18 that really the simplest reciprocating action
00:52:21 between oxygen and the end product of oxidative metabolism
00:52:25 that you, nature, could have designed.
00:52:28 And you wonder why nature hasn't actually used
00:52:32 that very simple method anywhere else.
00:52:34 You see, so here, oxygen expels bicarbonate,
00:52:41 and bicarbonate ion expels oxygen directly.
00:52:46 And you may wonder why the crocodilians want this.
00:52:52 Now, the explanation apparently is
00:52:55 that when a crocodile decides to eat you,
00:52:59 it does not tear you to pieces,
00:53:02 but he holds you underwater until you are drowned,
00:53:06 and then eats you up at his leisure.
00:53:09 Because he can stay underwater much longer than you can.
00:53:14 A crocodile can stay submerged for as much as an hour.
00:53:18 And so, he can do this
00:53:23 because this physiology of his hemoglobin
00:53:29 enables the crocodile to just extract
00:53:33 the last bit of oxygen from its blood.
00:53:40 Whereas a human usually can't extract
00:53:44 more than about a third of the oxygen.
00:53:48 There are other mechanisms.
00:53:50 Crocodilians can also cut off the circulation from their limbs
00:53:56 and only their brain and their viscera are oxygenated.
00:54:02 But that's another matter.
00:54:04 So, the question was, how does this work?
00:54:07 Now, can we go back a few slides, please?
00:54:12 Yes, further. Further. Further. Further.
00:54:18 Right.
00:54:21 The amino acid sequence is determined
00:54:24 by Leclerc and Schneck and Boissel
00:54:28 together with Raditz and Junig
00:54:31 showed the following replacement.
00:54:34 This histidine here was replaced by a proline
00:54:41 and the valine one by a serine.
00:54:45 The lysine was preserved.
00:54:48 The histidine replaced by a serine
00:54:52 and finally the glycine 144,
00:54:56 which is a variant of mammals,
00:54:58 was replaced by glutamate.
00:55:04 Christian Bauer, the German physiologist who did that work,
00:55:08 found that the crocodilian deoxy was a response
00:55:13 to bicarbonate ions per tetramer.
00:55:16 So, he set about the job in exactly the same way as with ATP.
00:55:21 He took the human hemoglobin model,
00:55:24 checked it rigidly,
00:55:26 nearly replaced the key residues to the proline here,
00:55:31 which made the chain turn by right angle
00:55:34 and put the N-terminal residue out there,
00:55:39 replaced this lysine here by glutamate
00:55:45 and this histidine by serine.
00:55:49 So, the last stereo slide shows you the result.
00:56:16 Here is a bicarbonate ion
00:56:20 and over here is the other.
00:56:25 So, you see, I take this replacement,
00:56:28 I put in two bicarbonate ions
00:56:30 and what I found, to my surprise,
00:56:33 was that one bicarbonate oxidant
00:56:39 could accept hydrogen bonds from the serine OH
00:56:43 in the alpha-amino group.
00:56:45 The second oxidant could accept the hydrogen bonds from the lysine
00:56:51 and the third could connect the hydrogen bonds
00:56:54 to the glutamate that had replaced lysine-144.
00:57:01 So, you see, again, by doing nothing to the model
00:57:05 but replacing these two key residues,
00:57:09 I was able to turn a diphosphoglycerate binding site
00:57:17 into a pair of binding sites for two bicarbonate ions.
00:57:40 Thank you.
00:57:43 Now, the patterns in mammals.
00:57:46 The most striking adaptive phenomena you can see
00:57:50 are adaptation to high altitude.
00:57:53 Can we again go...
00:57:55 Yes, at this slide, fine.
00:57:57 In the camel and the llama,
00:58:00 two closely related species,
00:58:03 but the llama, living at high altitude,
00:58:07 has a hemoglobin with high oxygen activity
00:58:10 and you ask yourself why.
00:58:13 Can we go back one more slide, please?
00:58:17 It turns out that the camel has a glutamine here
00:58:23 which can reach it nicely
00:58:26 to form a hydrogen bond with the phosphate,
00:58:29 but the llama has an asparagine
00:58:31 whose side chain is shorter than 1.3 angstroms
00:58:35 so that it just does not quite reach.
00:58:39 Hence, the affinity for diphosphoglycerate is lower
00:58:45 and the oxygen affinity is higher.
00:58:51 And there are several...
00:58:54 There are two species of geese.
00:59:00 The...
00:59:03 I was going to speak about mammals and now come to birds.
00:59:07 There are two species of geese.
00:59:10 The green-eyed goose that lives in the plains of India
00:59:13 and the bar-headed goose that migrates at 9,000 meters
00:59:18 across the tops of the Himalayas.
00:59:21 The bar-headed goose has a hemoglobin with high oxygen affinity
00:59:26 and there are only a few reactive substitutions.
00:59:35 Again, the answer is not so clear.
00:59:38 Probably, there is a replacement of one residue
00:59:43 which stabilizes the T-structure by a randomized contact
00:59:47 which is replaced in the bar-headed goose by another
00:59:51 that cannot make this contact.
00:59:54 But this is much less obvious and simple than the replacement here.
01:00:01 Now, finally, what happens in humans
01:00:08 when they climb to higher altitudes?
01:00:12 This problem began to interest the geologists again
01:00:18 when Messner and another, they wrote in
01:00:22 climb Everest without oxygen.
01:00:24 When everybody had said it was impossible to climb Everest without oxygen.
01:00:29 So, there has been an American expedition to Everest
01:00:34 where people actually climbed on top of the top
01:00:38 and took blood samples
01:00:40 and the measurements of venous CO2 pressure
01:00:46 with venous oxygen pressure
01:00:49 were made to see how it affected them.
01:00:54 Now, in humans, as we noticed before,
01:00:59 that are going to higher altitudes,
01:01:02 there's an increase in the concentration of red cell DPG
01:01:09 and this was thought to be an adaptive response
01:01:12 until it was discovered that in all animals and birds
01:01:16 that live at higher altitudes,
01:01:18 in fact, an increased oxygen affinity is adaptive.
01:01:23 Whereas in humans, it was thought that an increased concentration of DPG
01:01:28 allows the tissues to extract more oxygen from the blood
01:01:34 because the increased tTG would lower the oxygen affinity of hemoglobin.
01:01:39 So, it's very interesting to see what happens with these Everest climbers.
01:01:44 They did have an increased concentration of DPG
01:01:48 but the lowering of the oxygen affinity which that produced
01:01:52 was far outweighed by another effect.
01:01:56 Whereas in people at sea level,
01:02:01 the partial pressure of CO2, the venous CO2 pressure, is 40%.
01:02:06 In these Everest climbers, it had dropped to 7% by hyperventilation
01:02:13 as a result of which the pH of the blood has risen from 7.4 to 7.9
01:02:19 which produced such a large shift of the oxygen equilibrium curve
01:02:24 that the increased DPG level had no effect.
01:02:29 Apparently, it is that left shift which is adaptive
01:02:33 and allows people to reach those great heights.
01:02:37 Now, let me sum up.
01:02:41 From the examples I have shown you,
01:02:44 it looks as though adaptive changes are brought about
01:02:49 by pure amino acid substitution in key positions
01:02:53 rather producing large changes in chemical affinity
01:02:57 rather than by many substitutions producing small changes
01:03:01 so that my resulting studies have driven me to the same conclusion
01:03:08 that Kimura, the Japanese geneticist,
01:03:12 arrived at by entirely different arguments based on population genetics
01:03:18 namely that the majority of amino acid substitutions between species
01:03:23 are neutral as a result of genetic shifts rather than Darwinian selection
01:03:29 and that those groups in Darwinian selection are relatively few.
01:03:33 Thank you.
01:03:44 Those who are leaving now, please be sure to return your glasses.
01:03:49 It's very easy to walk out of the back step.